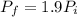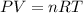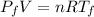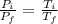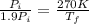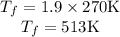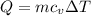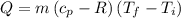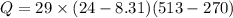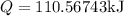Physics, 18.01.2020 00:31 RickyGotFanz4867
Asealed tank contains 29 moles of an ideal gas at an initial temperature of the pressure of the gas is increased until the final pressure equals 1.9 times the initial pressure. the heat capacity at constant pressure of the gas is what is the heat absorbed by the gas? let the ideal-gas constant r = 8.314 j/(mol • k).
7.0 kj
170 kj
230 kj
110 kj
-52 kj

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 01:20, Kjcampbell2
Your target variable is δf, the magnitude of the difference in frequency between the waves emitted from the sonar device and the waves received by the device after reflecting off the whale. write an expression for δf in terms of the relevant frequencies using the subscript notation introduced above.
Answers: 3


Physics, 22.06.2019 11:50, sillslola816oxb5h7
The scalar triple product computes the magnitude m of the moment of a force vector f about a specified line. it is m = (r × f) · n, where r is the position vector from the line to the point of application of the force and n is a unit vector in the direction of the line. use matlab to compute the magnitude m for the case where f = [12, −5, 4] n, r = [−3, 5, 2] m, and n = [6, 5, −7].
Answers: 3

Physics, 22.06.2019 14:50, MoogleCaliS
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 121.14 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/s. show the process on a t-s diagram.
Answers: 2
You know the right answer?
Asealed tank contains 29 moles of an ideal gas at an initial temperature of the pressure of the gas...
Questions in other subjects:



English, 20.02.2020 02:55


Social Studies, 20.02.2020 02:55


History, 20.02.2020 02:55

Mathematics, 20.02.2020 02:55

Mathematics, 20.02.2020 02:56

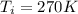
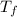 , the initial pressure will be
, the initial pressure will be 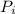 and the final pressure will be,
and the final pressure will be,