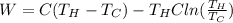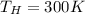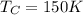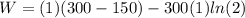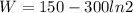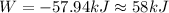Answers: 3


Other questions on the subject: Physics

Physics, 23.06.2019 00:00, meadowsoares7
What word chemical equation describes cavendish’s experiment with zinc?
Answers: 1

Physics, 23.06.2019 01:30, antoniocapetillo80
Sally turns on her cellular telephone to speak to her friend who is located thousands of miles away. which of the following best describes how such a telephone is able to transmit and receive information? a. the cellular telephone transmits, receives, and encodes information using only sound waves. b. the cellular telephone transmits, receives, and encodes information using only electromagnetic waves. c. the cellular telephone transmits information by electromagnetic waves to a receiver which then encodes them and produces sound. d. the cellular telephone transmits information by sound waves to a receiver which then encodes them and produces electromagnetic waves.
Answers: 2

Physics, 23.06.2019 12:30, banna01man
The lid of a pressure cooker forms a nearly airtight seal. steam builds up pressure and increases temperature within the pressure cooker so that food cooks faster than it does in an ordinary pot. the system is defined as the pressure cooker and the water and steam within it. if 2.0 g of water is sealed in a pressure cooker and then vaporized by heating, and 5127 j must be added as heat to completely vaporize the water, what is the change in the system’s internal energy? answer in units of j.
Answers: 2
You know the right answer?
Calculate the work performed by an ideal carnot engine as a cold brick warms from 150 k to the tempe...
Questions in other subjects:


Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10


History, 04.12.2020 22:10

Spanish, 04.12.2020 22:10

Biology, 04.12.2020 22:10

History, 04.12.2020 22:10

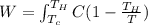
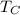 = Cold Temperature
= Cold Temperature 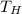 = Hot Temperature
= Hot Temperature