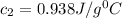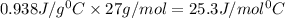Physics, 24.12.2019 21:31 jamiezanfardino1464
A23.5 g piece of aluminum metal is initially at 100.0°c. it is dropped into a coffee cup-calorimeter containing 130.0 g of water at a temperature of 23.0°c. after stirring, the final temperature of both copper and water is 26.0°c. assuming no heat losses, and that the specific heat capacity of water is 4.184 j/(g·°c), what is the molar heat capacity of aluminum, cm(al)?

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 19:30, yolinda123429
Molten iron fills a mould, which has a volume of 200 cm cubed. calculate the volume when the iron cools and solidifies.
Answers: 1


You know the right answer?
A23.5 g piece of aluminum metal is initially at 100.0°c. it is dropped into a coffee cup-calorimeter...
Questions in other subjects:

Mathematics, 13.02.2021 20:50

Mathematics, 13.02.2021 20:50


Biology, 13.02.2021 20:50

Computers and Technology, 13.02.2021 20:50


English, 13.02.2021 20:50

Mathematics, 13.02.2021 20:50

Mathematics, 13.02.2021 20:50

Mathematics, 13.02.2021 20:50

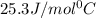
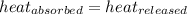
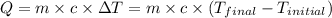
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0432/0719/09236.png) .................(1)
.................(1)
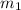 = mass of water = 130.0 g
= mass of water = 130.0 g
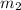 = mass of aluminiunm = 23.5 g
= mass of aluminiunm = 23.5 g
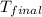 = final temperature
=
= final temperature
= 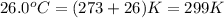
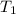 = temperature of water =
= temperature of water = 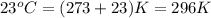
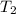 = temperature of aluminium =
= temperature of aluminium = 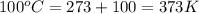
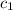 = specific heat of water=
= specific heat of water= 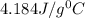
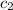 = specific heat of aluminium= ?
= specific heat of aluminium= ?![130.0\times 4.184\times (299-296)=-[23.5\times c_2\times (299-373)]](/tpl/images/0432/0719/5591a.png)