
Physics, 20.12.2019 02:31 tystevens9943
Atomic hydrogen produces a well-known series of spectral lines in several regions of the electromagnetic spectrum. each series fits the rydberg equation with its own particular n1 value. calculate the value of n1 that would produce a series of lines in which the highest energy line has a wavelength of 821 nm.

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 11:40, Destine1965
Consider the following position function. find (a) the velocity and the speed of the object and (b) the acceleration of the object. bold r left parenthesis t right parenthesisr(t)equals=left angle 6 t superscript 4 baseline comma 2 t cubed right angle6t4,2t3 for tgreater than or equals≥0
Answers: 3


Physics, 22.06.2019 12:50, keilahsalmon
The vapour pressure of benzene is 53.3 kpa at 60.6 °c, but it fell to 51.5 kpa when 19.0 g of a non-volatile organic compound was dissolved in 500 g of benzene. calculate the molar mass of the compound.
Answers: 2

Physics, 22.06.2019 21:00, andybiersack154
The velocity of a car traveling in a straight line increases from 0 meters/second to 30meters/second in 8 seconds. what is the average acceleration of the car?
Answers: 1
You know the right answer?
Atomic hydrogen produces a well-known series of spectral lines in several regions of the electromagn...
Questions in other subjects:

Computers and Technology, 18.12.2020 17:00




Mathematics, 18.12.2020 17:00





History, 18.12.2020 17:00

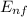 - E₀ = - k²e² / 2m (1 /
- E₀ = - k²e² / 2m (1 / 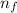 ²2 - 1 / n₀²)
²2 - 1 / n₀²)
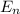 (eV)
(eV) 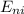 (eV)
(eV)


