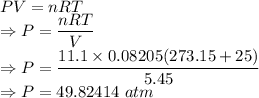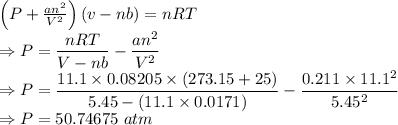Physics, 20.12.2019 01:31 moisealafleur
Calculate the pressure exerted by 11.1 moles of neon gas in a volume of 5.45 l at 25°c using (a) the ideal gas equation and (b) the van der waals equation. (for neon, a = 0.211 atm · l2/mol2 and b = 0.0171 l/mol.)

Answers: 2


Other questions on the subject: Physics


Physics, 22.06.2019 10:10, princeofpowerjr
Which branches of natural science include the study of an organism that lived 10 million years ago
Answers: 1


Physics, 22.06.2019 23:10, Jgarciar541
Atorque acting on an object tends to produce a. equilibrium b. rotation c. linear motion d. velocity e. a center of gravity
Answers: 2
You know the right answer?
Calculate the pressure exerted by 11.1 moles of neon gas in a volume of 5.45 l at 25°c using (a) the...
Questions in other subjects:



English, 05.05.2020 22:16




Mathematics, 05.05.2020 22:16


Computers and Technology, 05.05.2020 22:16