
Physics, 19.12.2019 06:31 kaywendel2008
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula: =e−ryn2 in this equation ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron. (you can find the value of the rydberg energy using the data button on the aleks toolbar.) calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with =n10 to an orbital with =n8. round your answer to 3 significant digits.

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 15:30, jonathanmagana112002
Charge is distributed along the entire x-axis with uniform density λ. how much work does the electric field of this charge distribution do on an electron that moves along the y-axis from y = a to y = b? (use the following as necessary: a, b, ε0, λ, and q for the charge on an electron.)
Answers: 3

Physics, 22.06.2019 16:50, Walkman2092
Calculate the first and second velocities of the car with four washers attached to the pulley, using the formulas v1 = 0.25 m / t1 , and v2 = 0.25 m / (t2 – t1) where t1 and t2 are the average times the car took to reach the 0.25 and the 0.50 meter marks. record these velocities, to two decimal places, in table e.
Answers: 2

Physics, 22.06.2019 19:30, dragador7601
Which of the following compounds would be primarily ionic? methane, ch4, ammonia nh3, calcium chloride, cacl2, or carbon dioxide, co2
Answers: 1
You know the right answer?
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula: =e−ryn2 in...
Questions in other subjects:




Mathematics, 05.09.2020 07:01






Mathematics, 05.09.2020 07:01

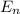 = - k²e² / 2m (1 / n²)
= - k²e² / 2m (1 / n²)
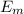 = - k²e² / 2m (1 /
= - k²e² / 2m (1 / 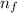 ² -1 /
² -1 / 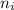 ²)
²)


