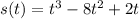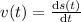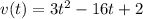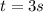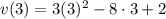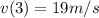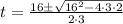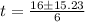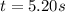Physics, 18.12.2019 21:31 BrodsterBj
Aparticle moves according to the law of motion s(t) = t^{3}-8t^{2}+2t, t\ge 0, where t is measured in seconds and s in feet. a.) find the velocity at time t. b.) what is the velocity after 3 seconds?c.) when is the particle at rest? enter your answer as a comma separated list. enter none if the particle is never at rest. at t_1= and t_2= with t_1

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 00:00, tsupreme
1134567 a jet with mass m = 1.1 ă— 105 kg jet accelerates down the runway for takeoff at 2 m/s2. 1) what is the net horizontal force on the airplane as it accelerates for takeoff? 2.2*10^5 n your submissions: 2.2*10^5 computed value: 220000submitted: saturday, january 26 at 2: 41 pm feedback: correct! 2) what is the net vertical force on the airplane as it accelerates for takeoff? 0 n your submissions: 0 computed value: 0submitted: saturday, january 26 at 2: 41 pm feedback: correct! 3) once off the ground, the plane climbs upward for 20 seconds. during this time, the vertical speed increases from zero to 21 m/s, while the horizontal speed increases from 80 m/s to 95 m/s. what is the net horizontal force on the airplane as it climbs upward? n 4) what is the net vertical force on the airplane as it climbs upward? n 5) after reaching cruising altitude, the plane levels off, keeping the horizontal speed constant, but smoothly reducing the vertical speed to zero, in 13 seconds. what is the net horizontal force on the airplane as it levels off? n 6) what is the net vertical force on the airplane as it levels off?
Answers: 1


Physics, 22.06.2019 05:50, kamrulh278
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 08:30, ljwatts25
Pl asaaap ! match the term to the correct description. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. movement that is like a t transfers energy from one location to another 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1
You know the right answer?
Aparticle moves according to the law of motion s(t) = t^{3}-8t^{2}+2t, t\ge 0, where t is measured i...
Questions in other subjects:





History, 17.07.2019 12:40

Physics, 17.07.2019 12:40


Mathematics, 17.07.2019 12:40

History, 17.07.2019 12:40