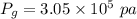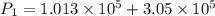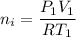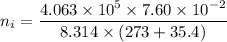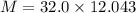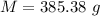Physics, 17.12.2019 04:31 AnActualTrashcan
Awelder using a tank of volume 7.60×10−2 m3 fills it with oxygen (with a molar mass of 32.0 g/mol ) at a gauge pressure of 3.05×105 pa and temperature of 35.4 ∘c. the tank has a small leak, and in time some of the oxygen leaks out. on a day when the temperature is 20.8 ∘c, the gauge pressure of the oxygen in the tank is 1.95×105 pa. find the initial mass of oxygen. (in kg)

Answers: 3


Other questions on the subject: Physics


Physics, 22.06.2019 13:20, Tess3492
It is reasonable to assume that the bulk modulus of blood is about the same as that of water (2.2 gpa). as one goes deeper and deeper in the ocean, the pressure increases by 10000 pa for every meter below the surface. if a diver goes down 80.0 m in the ocean, by how much does each cubic centimeter of her blood change in volume? give the answer in cubic centimeters (actually one cubic centimeter equals one milliliter).
Answers: 2

Physics, 22.06.2019 18:30, Jasten
Arailroad car collides with and sticks to an identical railroad car that is initially at rest. after the collision, the total kinetic energy of the two cars is a) the same as before. b) half as much as before. c) one third as much as before. d) one fourth as much as before. e) twice as much as before.
Answers: 1
You know the right answer?
Awelder using a tank of volume 7.60×10−2 m3 fills it with oxygen (with a molar mass of 32.0 g/mol )...
Questions in other subjects:

Biology, 14.07.2020 20:01




Mathematics, 14.07.2020 20:01