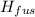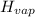Physics, 17.12.2019 01:31 Paulalex8765
How much heat energy is required to vaporize a 1.0-g ice cube at 0°c? the heat of fusion of ice is 80 cal/g.
the heat of vaporization of water is 540 cal/g, and cwater = 1.00 cal/g⋅°c.
a. 620 cal
b. 720 cal
c. 820 cal
d. 1 kcal

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 21:00, danidavis2002
In order to place a satellite into orbit, it requires enough fuel to supply the necessary mechanical energy. into what types of mechanical energy does the fuel get transformed?
Answers: 1

Physics, 22.06.2019 02:30, ntangpricha
Eddy whose mass is 55kg climbs up the 1.50 meter high stairs in 2s. calculate eddy’s power rating
Answers: 1

Physics, 22.06.2019 12:00, louieknown
Suppose a wire with a current is pushed upward by a magnetic field. how would the wire move if the direction of the current reversed? explain your answer.
Answers: 1

Physics, 22.06.2019 22:30, gokusupersaiyan12345
Find v1, the speed of the fluid in the left end of the main pipe. express your answer in terms of h1, h2, g, and either a1 and a2 or γ, which is equal to a1a2.
Answers: 2
You know the right answer?
How much heat energy is required to vaporize a 1.0-g ice cube at 0°c? the heat of fusion of ice is...
Questions in other subjects:






Mathematics, 01.09.2020 23:01


History, 01.09.2020 23:01