
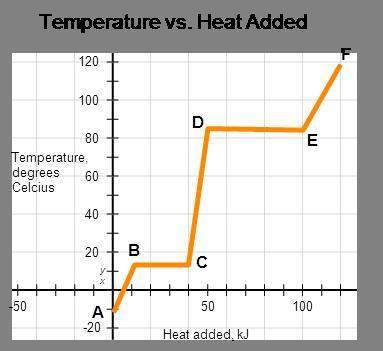
The represented substance has the following heat capacities, enthalpy of fusion, and enthalpy of vaporization.
cp, solid = 3.2 j/(g•°c); cp, liquid = 5.3 j/(g•°c);
cp, vapor = 8.9 j/(g•°c); δhfus = 4.5 kj/mol;
δhvap = 8.6 kj/mol
calculate the total energy input required to accomplish vaporization of one mole of substance at 15°c. the molar mass of the substance is 120.0 g/mol.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 18:00, Pumpkinputters
Awind turbine is initially spinning at a constant angular speed. as the wind's strength gradually increases, the turbine experiences a constant angular acceleration 0.173 rad/s2. after making 2870 revolutions, its angular speed is 132 rad/s. (a) what is the initial angular velocity of the turbine? (b) how much time elapses while the turbine is speeding up?
Answers: 1

Physics, 22.06.2019 23:30, danielahchf
Which of the following forces require physical contact? tension air resistance friction magnetic force applied force
Answers: 3


Physics, 23.06.2019 03:30, allieeastridge
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
The represented substance has the following heat capacities, enthalpy of fusion, and enthalpy of vap...
Questions in other subjects:




Chemistry, 27.01.2021 08:30



History, 27.01.2021 08:30



English, 27.01.2021 08:30



