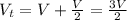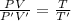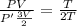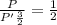Physics, 14.12.2019 06:31 sierraseideman1023
Asystem of ideal gas has an initial pressure of 111 kpa and occupies a volume of 5.00 liters. doubling the system’s absolute temperature by means of a constant-pressure process would require an amount of work w. instead, you decide to double the absolute temperature by carrying out two processes in sequence, a constant-pressure process followed by a constant-volume process. in this case, the total work done in the two-process sequence is w/2. calculate the final pressure of the system.

Answers: 3


Other questions on the subject: Physics


Physics, 23.06.2019 05:10, richardharding
For those who need theses answers i just took the test and got everyone of them
Answers: 3

Physics, 23.06.2019 06:30, acharity196
Acopper sheet has a volume of 100 cm³. if the density of copper is 8.9 g/cm³, what is the mass of the sheet? a. 11 g b. 8.9 g c. 189 g d. 890 g
Answers: 1
You know the right answer?
Asystem of ideal gas has an initial pressure of 111 kpa and occupies a volume of 5.00 liters. doubli...
Questions in other subjects:


Business, 29.01.2020 16:43

Spanish, 29.01.2020 16:43

English, 29.01.2020 16:43

Computers and Technology, 29.01.2020 16:43

Mathematics, 29.01.2020 16:43


Mathematics, 29.01.2020 16:44


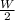
 (2)
(2)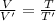
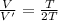
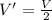
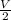 given by:
given by: