
Physics, 14.12.2019 05:31 moraleserica011
You read in a popular diet blog that the author claims you can lose up to half a pound of body fat per week if, instead of drinking 1.7 liters (i. e., 1.7 kilograms) of water every day, you can eat the same amount of water in the form of ice. the reasoning is that your body must first melt the ice and then bring the ice water up to the body temperature of 37°c, which is a process that requires a significant amount of energy at the expense of "burned" body fat. , you just finished discussing heat and phase changes in your physics class, so you can calculate exactly how much energy is expended. the specific heat and latent heat of fusion for water are 4.186 x 10^3 j/(kg: °c) and 3.34x10^9 j/kg, respectively.
(a) how many kilocalories do you expend when you eat 1.7 kg of ice at 0°c? note that 1 kcal = 4186 j.
(b) assuming the metabolism of one pound of body fat produces 3500 kcal of energy, how many pounds of body fat could you lose in a week just by eating 1.7 kilograms of ice every day?

Answers: 1


Other questions on the subject: Physics


Physics, 22.06.2019 07:20, coolusername1314
The softest and hardest minerals are: softest hardest
Answers: 1

Physics, 22.06.2019 11:20, ashtonbillups
More solar radiation is absorbed by earth’s surface than by
Answers: 1
You know the right answer?
You read in a popular diet blog that the author claims you can lose up to half a pound of body fat p...
Questions in other subjects:



Social Studies, 20.09.2020 19:01


Physics, 20.09.2020 19:01

Law, 20.09.2020 19:01




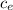 ΔT = m
ΔT = m 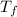 –T₀)
–T₀) 

