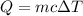Physics, 18.09.2019 03:00 asseatingbandit
If the specific heat of water is 1.0 calorie/gram°c, how many calories are required to raise 500 grams of water 10.0°c? 510 cal 5,000 cal 50 cal 501 cal

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 11:30, azduce
(2) (a) you have a simple circuit that consists of only a battery (δvbat=1.5v) and two resistors with resistance r1=10ω and r2=5ω, connected in series with each other. what is the current running through the battery? (b) you re-arrange your circuit so now r2 is attached in parallel to r1 rather than in series. what is the current running through the battery? (c) you add an additional resistor r3=7ω on the same branch as r2. what is the current running through the battery? (d) what is the power dissipated in r3?
Answers: 3

Physics, 22.06.2019 12:30, lanaasad7733
An ice-making machine inside a refrigerator operates in a carnot cycle. it takes heat from liquid water at 0.0 degrees celsius and rejects heat to a room at a temperature of 19.2 degrees celsius. suppose that liquid water with a mass of 76.3kg at 0.0 degrees celsius is converted to ice at the same temperature. take the heat of fusion for water to be l_f = 3.34*10^5 j/kg. how much energy e must be supplied to the device? express your answer in joules.
Answers: 1

Physics, 22.06.2019 13:50, highlander4215
Observations show that interstellar clouds can have almost any shape and
Answers: 1
You know the right answer?
If the specific heat of water is 1.0 calorie/gram°c, how many calories are required to raise 500 gra...
Questions in other subjects:


Mathematics, 29.05.2021 07:00

Mathematics, 29.05.2021 07:00



Biology, 29.05.2021 07:00

Social Studies, 29.05.2021 07:00