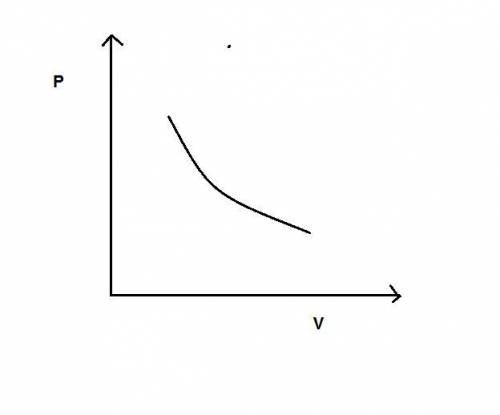
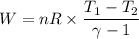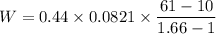During an adiabatic expansion the temperature of 0.440 mol of argon (ar) drops from 61.0 ∘c to 10.0 ∘c. the argon may be treated as an ideal gas.(a) draw a pv-diagram for this process.
(b) how much work does the gas do?
(c) what is the change in internal energy of the gas? explain.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 09:00, winterblanco
The pressure proportional to the area a- inversely b- directly c- increase d-decrease
Answers: 2

Physics, 22.06.2019 12:50, keilahsalmon
The vapour pressure of benzene is 53.3 kpa at 60.6 °c, but it fell to 51.5 kpa when 19.0 g of a non-volatile organic compound was dissolved in 500 g of benzene. calculate the molar mass of the compound.
Answers: 2


Physics, 22.06.2019 20:30, cupcake3103670
Aball is thrown from the top of a building with an initial velocity of 21.9 m/s straight upward, at an initial height of 51.6 m above the ground. the ball just misses the edge of the roof on its way down, as shown in the figure. (a) determine the time needed for the ball to reach its maximum height. (b) determine the maximum height. (c) determine the time needed for the ball to return to the height from which it was thrown, and the velocity of the ball at that instant. (d) determine the time needed for the ball to reach the ground. (e) determine the velocity and position of the ball at t = 5.35 s.
Answers: 1
You know the right answer?
During an adiabatic expansion the temperature of 0.440 mol of argon (ar) drops from 61.0 ∘c to 10.0...
Questions in other subjects:


Chemistry, 03.10.2021 14:40

Mathematics, 03.10.2021 14:40

Mathematics, 03.10.2021 14:40

Biology, 03.10.2021 14:40


Mathematics, 03.10.2021 14:40

Mathematics, 03.10.2021 14:40


Chemistry, 03.10.2021 14:40