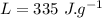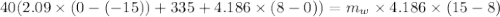A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) containing water at 15.00°c.
when equilibrium is reached, the final temperature is 8.00°c.
how much water did the calorimeter contain initially?
the specific heat of ice is 2090 j/kg • k, that of water is 4186 j/kg • k, and the latent heat of fusion of water is 33.5 × 104 j/kg.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 14:30, darceline1574
Alandscaper is shopping for landscaping materials. she wants to use materials through which water flows easily. which materials should she choose? check all that apply. clay gravel granite rocks with cracks loosely packed soil
Answers: 2

Physics, 22.06.2019 18:30, Jasten
Arailroad car collides with and sticks to an identical railroad car that is initially at rest. after the collision, the total kinetic energy of the two cars is a) the same as before. b) half as much as before. c) one third as much as before. d) one fourth as much as before. e) twice as much as before.
Answers: 1

Physics, 22.06.2019 22:50, mdg5605
The illuminance of a surface varies inversely with the square of its distance from the light source. if the illuminance of a surface is 120 lumens per square meter when its distance from a certain light source is 6 meters, by how many meters should the distance of the surface from the source be increased to reduce its illuminance to 30 lumens per square meter?
Answers: 3
You know the right answer?
A40.0-g block of ice at -15.00°c is dropped into a calorimeter (of negligible heat capacity) contain...
Questions in other subjects:

Mathematics, 02.02.2021 22:20



History, 02.02.2021 22:20


Social Studies, 02.02.2021 22:20

History, 02.02.2021 22:20

Mathematics, 02.02.2021 22:20

Mathematics, 02.02.2021 22:20

English, 02.02.2021 22:20


 initial temperature of ice block,
initial temperature of ice block,  initial temperature of water,
initial temperature of water,  final temperature of mixture,
final temperature of mixture,  specific heat of ice,
specific heat of ice, 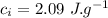 specific heat of water,
specific heat of water,  Latent heat of fusion of water,
Latent heat of fusion of water,