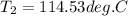Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 22:00, natethawsm
Na food processing facility, a spherical container of inner radius r1 5 40 cm, outer radius r2 5 41 cm, and ther- mal conductivity k 5 1.5 w/m · k is used to store hot water and to keep it at 100°c at all times. to accomplish this, the outer surface of the container is wrapped with a 800-w electric strip heater and then insulated. the temperature of the inner surface of the container is observed to be nearly 120°c at all times. as- suming 10 percent of the heat generated in the heater is lost through the insulation, (a) express the differential equation and the boundary conditions for steady one-dimensional heat conduction through the container, (b) obtain a relation for the variation of temperature in the container material by solving the differential equation, and (c) evaluate the outer surface tempera- ture of the container. also determine how much water at 100°c this tank can supply steadily if the cold water enters at 20°c.
Answers: 2


Physics, 22.06.2019 12:20, ginachuquiano450
Aball with a mass of 275 g is dropped from rest, hits the floor and rebounds upward. if the ball hits the floor with a speed of 2.40 m/s and rebounds with a speed of 1.70 m/s, determine the following. (a) magnitude of the change in the ball's momentum (let up be in the positive direction.)
Answers: 1

Physics, 22.06.2019 13:00, Ryleetarver
Discuss how the hardness or softness of the landing surface is related to the time required to stop the egg
Answers: 1
You know the right answer?
We start with 5.00 moles of an ideal monatomic gas with an initial temperature of 128 ∘c. the gas ex...
Questions in other subjects:


English, 31.03.2020 21:17



Mathematics, 31.03.2020 21:17