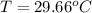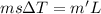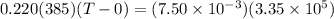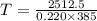Physics, 07.12.2019 00:31 herchellann302
You place an ice cube of mass 7.50×10−3kg and temperature 0.00∘c on top of a copper cube of mass 0.220 kg. all of the ice melts, and the final equilibrium temperature of the two substances is 0.00∘c. what was the initial temperature of the copper cube? assume no heat is exchanged with the surroundings.

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 21:50, porkhappycom
It is may 5 and you are somewhere in the northern hemisphere. if you determine that the noon sun is 51 degrees above your southern horizon, what is the latitude of your location
Answers: 1

Physics, 22.06.2019 05:30, dontcareanyonemo
What are similarities and differences of reflection, refraction, diffraction and absorption?
Answers: 1

Physics, 22.06.2019 07:00, lilpeepxliltracy
Aball has an initial velocity of 3 m/s. if there is no friction, what is the highest it could roll?
Answers: 1

Physics, 22.06.2019 18:30, andy6128
Energy can be transformed from one form to another. the diagram shows one such process. which energy transformation is represented in the diagram? nuclear to thermal and radiant nuclear to electrical and chemical chemical to nuclear and radiant chemical to electrical and nuclear
Answers: 1
You know the right answer?
You place an ice cube of mass 7.50×10−3kg and temperature 0.00∘c on top of a copper cube of mass 0.2...
Questions in other subjects:

Mathematics, 28.10.2020 01:00

Mathematics, 28.10.2020 01:00

Social Studies, 28.10.2020 01:00


Mathematics, 28.10.2020 01:00





Mathematics, 28.10.2020 01:00