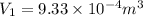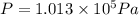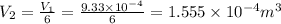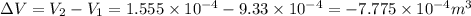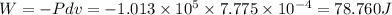Physics, 02.12.2019 22:31 melaniegilbreath
Consider a cylinder initially filled with 9.33 10-4 m3 of ideal gas at atmospheric pressure. an external force is applied to slowly compress the gas at constant temperature to 1/6 of its initial volume. calculate the work that is done. note that atmospheric pressure is 1.013 105 pa.

Answers: 1


Other questions on the subject: Physics


Physics, 22.06.2019 16:40, dylan6981
The astronauts on the space shuttle flights experienced an acceleration of 29 m/s2 (about 3 "g's") during lift-off. what upward force must the astronaut's seat apply to the astronaut in order to cause this acceleration? assume the astronaut's mass is 70 kg, and compute this force when the acceleration is near the earth's surface so their weight equals latex: mg m g .
Answers: 3

Physics, 22.06.2019 17:30, ciaotaylor
Aball thrown by ginger is moving upward through the air. diagram a shows a box with a downward arrow. diagram b shows a box with an upward arrow. diagram c shows a box with a downward and upward arrow equal in size. diagram d shows a box with a downward and upward arrow with the downward arrow larger in size. which force diagram represents the forces on the ball? you may neglect the effects of air resistance.
Answers: 3

Physics, 22.06.2019 20:50, dorkygirl
An ideal otto cycle has a compression ratio of 8. at the beginning of the compression process, air is at 95 kpa and 27°c, and 750 kj/kg of heat is transferred to air during the constant-volume heat-addition process. assuming constant specific heats at room temperature, determine (a) the pressure and temperature at the end of the heat-addition process, (b) the net work output, (c) the thermal efficiency, and (d) the mean effective pressure for the cycle. (4390 kpa, 1730 k; 423 kj/kg; 56.4%; 534 kpa)
Answers: 1
You know the right answer?
Consider a cylinder initially filled with 9.33 10-4 m3 of ideal gas at atmospheric pressure. an exte...
Questions in other subjects:







Mathematics, 13.06.2020 18:57