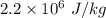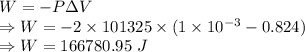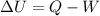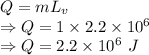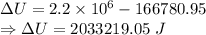Physics, 29.11.2019 04:31 lizdeleon248
When water is boiled under a pressure of 2.00atm, the heat of vaporization is 2.20×106j/kg and the boiling point is 120∘c. at this pressure, 1.00kg of water has a volume of 1.00×10−3m3, and 1.00 kg of steam has a volume of 0.824m3.
a)compute the work done when 1.00kg of steam is formed at this temperature
b)compute the increase in internal energy of the water.

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 22:30, cocodemain
A1200 kg car traveling north at 10 m/s is rear-ended by a 2000 kg truck traveling at 30 m/s. what is the total momentum before and after the collision?
Answers: 3

Physics, 21.06.2019 23:00, laurachealsy923
How many dots must be added to the symbol to properly represent a standard nitrogen ion? a) 1 b) 3 c) 5 d) 8
Answers: 1

Physics, 22.06.2019 04:30, cicimarie2018
Ameter stick is pivoted at the 0.50-m line. a 3.0-kg object is hung from the 0.15-m line. where should a 5.0-kg object be hung to achieve equilibrium (the meter stick oriented horizontal and motionless)?
Answers: 1

Physics, 22.06.2019 09:40, zackarygonzalez1028
Which is special about a dc circuit ? a. it has both series and parallel components b. it has only series or only parallel components c. charge moves in a single direction d. charge moves back and forth quickly
Answers: 3
You know the right answer?
When water is boiled under a pressure of 2.00atm, the heat of vaporization is 2.20×106j/kg and the b...
Questions in other subjects:


Geography, 14.10.2020 18:01


English, 14.10.2020 18:01

Mathematics, 14.10.2020 18:01




Mathematics, 14.10.2020 18:01

Mathematics, 14.10.2020 18:01

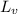 = Heat of vaporization =
= Heat of vaporization =