Which of the following is not an assumption of the kinetic molecular theory for a gas?
a. ga...

Which of the following is not an assumption of the kinetic molecular theory for a gas?
a. gases are made up of tiny particles in constant chaotic motion.
b. gas particles are very small compared to the average distance between the particles.
c. gas particles collide with the walls of their container in elastic collisions.
d. the average velocity of the gas particles is directly proportional to the mass of the gas particles at constant temperature.
e. all of the above are assumptions of the kinetic molecular theory.

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 19:30, ctuek5990
The 20@kg wheel has a radius of gyration about its center g of kg = 300 mm. when it is subjected to a couple moment of m = 50 n # m, it rolls without slipping. determine the angular velocity of the wheel after its mass center g has traveled through a distance of sg = 20 m, starting from rest.
Answers: 3

Physics, 22.06.2019 05:30, ninaaforever
Aforklift raises a 1,020 n crate 3.50 m up to a shelf. how much work is done by the forklift on the crate? the forklift does j of work on the crate.
Answers: 2


Physics, 22.06.2019 16:00, ggdvj9gggsc
Suppose a soccer ball is kicked from the ground at an angle 20.0º above the horizontal at 8.00 m/s. the y-velocity is determined to be 2.74 m/s. how long will the ball be in the air? assume the ball lands at the same height at which it was kicked.
Answers: 2
You know the right answer?
Questions in other subjects:



Mathematics, 07.04.2020 19:34





History, 07.04.2020 19:34


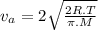
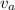 = average velocity of the gas particles
= average velocity of the gas particles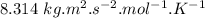 )
) )
)

