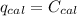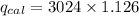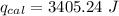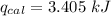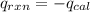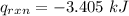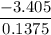Physics, 27.11.2019 01:31 CyberSongWriter
When 0.1375 g of solid magnesium is burned in a constant-volume bomb calorimeter, the temperature increases by 1.126°c. the heat capacity of the bomb calorimeter, determined in a separate experiment, is 3024 j/°c. calculate the heat given off by the burning magnesium in kj/mol.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 04:00, zoepatterson1p0lij1
When an object is heated it contracts. a. true b. false
Answers: 2

Physics, 22.06.2019 05:40, izzyisawesome5232
An ideal polarizer with its transmission axis rotated 30 degrees to the vertical is placed in a beam of unpolarized light of intensity 10w/m^2. after passing through the polarizer, what is the intensity of the beam? a. 8.7 w/m^2 b. 7.5 w/m^2 c. 5.0 w/m^2 d. 10 w/m^2 e. 2.5 w/m^2
Answers: 1

Physics, 22.06.2019 09:00, kiarajack456
Why can blue light and yellow light combine to produce white light? question 17 options: they are both primary colors of light. they absorb each other's wavelengths. because blue, yellow, and white are primary colors. they are complementary colors of light.
Answers: 1

Physics, 22.06.2019 11:00, dragon2565
Looking at this barometer, is the air pressure high or low? what type of weather would you expect? high, bad low, bad high, good low, good
Answers: 1
You know the right answer?
When 0.1375 g of solid magnesium is burned in a constant-volume bomb calorimeter, the temperature in...
Questions in other subjects:





Social Studies, 26.09.2019 13:30




History, 26.09.2019 13:30