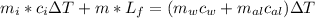Iplace an ice cube with a mass of 0.223 kg and a temperature of −35◦c is placed into an insulated aluminum container with a mass of 0.553 kg containing 0.452 kg of water. the water and the container are initially in thermal equilibrium tatemperature of 27◦c. system, whatwillthefinaltemperature of the system be when it reaches equilibrium, and how much ice will be in the container when it reaches equilibrium

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 04:40, joneil1952
Argon is adiabatically compressed from an initial volume of 16 liters to a final volume of 2 liters. by what factor do the following quantities change? do they increase or decrease? (a) the rms speed (b) the thermal energy of the gas (c) the molar specific heat cv (d) the pressure
Answers: 3


Physics, 23.06.2019 01:30, martinezkimberly706
Match each type of lever with the correct diagram. 1. first-class 2. third-class 3. second-class
Answers: 3
You know the right answer?
Iplace an ice cube with a mass of 0.223 kg and a temperature of −35◦c is placed into an insulated al...
Questions in other subjects:



Social Studies, 29.06.2021 22:00



English, 29.06.2021 22:00




Geography, 29.06.2021 22:10

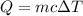
 Change in temperature
Change in temperature 
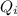 = Heat absorbed by whole ice
= Heat absorbed by whole ice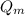 = Heat absorbed by mass
= Heat absorbed by mass = Heat energy by latent heat fusion/melting
= Heat energy by latent heat fusion/melting