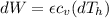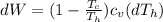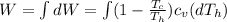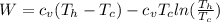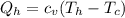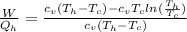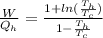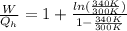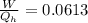Say that you are in a large room at temperature tc = 300 k. someone gives you a pot of hot soup at a temperature of th = 340 k. you set the bowl up so that as it cools to room temperature the heat first flows through a carnot engine. the soup has cv= (33 j/k). assume that the volume of the soup does not change. what fraction of the total heat qh that is lost by the soup can be turned into useable work by the engine? work / qh =

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 07:30, jaquezdaking4919
The slope of a velocity time graph over any interval of time gives the during that interval
Answers: 2


Physics, 22.06.2019 18:30, dakotakeating4513
Apiece of metal 75g at 110°c is dropped in a container with 500g at 20°c. the h2o if temperature is 24°c find specific heat capacity of metal
Answers: 3

Physics, 22.06.2019 23:00, shelbybibb99
Acommon technique in analysis of scientific data is normalization. the purpose of normalizing data is to eliminate irrelevant constants that can obscure the salient features of the data. the goal of this experiment is to test the hypothesis that the flux of light decreases as the square of the distance from the source. in this case, the absolute value of the voltage measured by the photometer is irrelevant; only the relative value conveys useful information. suppose that in part 2.2.2 of the experiment, students obtain a signal value of 162 mv at a distance of 4 cm and a value of 86 mv at a distance of 5.7 cm. normalize the students' data to the value obtained at 4 cm. (divide the signal value by 162.) then calculate the theoretically expected (normalized) value at 5.7 cm.
Answers: 2
You know the right answer?
Say that you are in a large room at temperature tc = 300 k. someone gives you a pot of hot soup at a...
Questions in other subjects:



World Languages, 11.07.2019 18:00

History, 11.07.2019 18:00




Biology, 11.07.2019 18:00

English, 11.07.2019 18:00

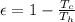
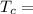 Temperature at the room
Temperature at the room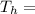 Temperature of the soup
Temperature of the soup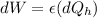
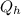 represents the input heat and at the same time is defined as
represents the input heat and at the same time is defined as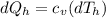
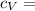 Specific Heat
Specific Heat