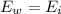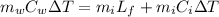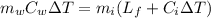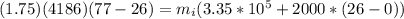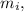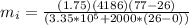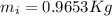Physics, 21.11.2019 18:31 weridness80
How much ice (at 0°c) must be added to 1.75 kg of water at 77 °c so as to end up with all liquid at 26 °c? (ci = 2000 j/(kg.°c), cw = 4186 j/(kg.°c), lf= 3.35 × 10 5 3.35×105 j/kg, lv= 2.26 × 10 6 2.26×106 j/kg)

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 04:00, tamyahamlin02p6b7yt
Determine the maximum r-value of the polar equation r =3+3 cos 0
Answers: 3

Physics, 22.06.2019 12:00, P4thing
Under the action of a constant force an object accelerates at 7.8 m/s2. what will the acceleration be if (a) the force is halved? (b) the object's mass is halved? (c) the force and the object's mass are both halved? (d) the force is halved and the object's mass is doubled?
Answers: 3


Physics, 22.06.2019 23:00, lesmontoya10
Identify the type of heat transfer occurring when chocolate melts in your hand
Answers: 1
You know the right answer?
How much ice (at 0°c) must be added to 1.75 kg of water at 77 °c so as to end up with all liquid at...
Questions in other subjects:




Mathematics, 12.07.2019 02:10


Mathematics, 12.07.2019 02:10

Mathematics, 12.07.2019 02:10



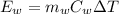
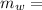 mass of water
mass of water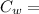 Specific Heat of Water
Specific Heat of Water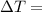 Change in Temperature
Change in Temperature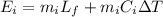
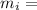 Mass of ice
Mass of ice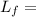 Latent heat of fusion
Latent heat of fusion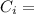 Specific heat of Ice
Specific heat of Ice