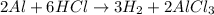Physics, 11.11.2019 08:31 caromaybelline71
How does the law of conservation of mass apply to this reaction: al + 3hcl → h2 + alcl3 ?
hydrogen and chlorine need to be balanced. there is an equal amount of aluminum on each side.
only the hydrogen needs to be balanced. there are equal numbers of aluminum and chlorine.
the law of conservation of mass has already been applied. there is an equal number of each element on both sides of the equation.
the equation needs to be balanced. there are fewer hydrogen atoms in the equation than aluminum or chlorine.

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 07:00, divadebbgirl1
Examine the equation. 23490th→23088ra+42he what kind of barrier would you need to block the radioactive particles from this reaction? a. a piece of paper b. a sheet of aluminum foil c. a two-inch block of lead d. a solid concrete block
Answers: 1

Physics, 22.06.2019 07:30, carlinryan
Which of the following is an example of motion in two dimensions?
Answers: 3


Physics, 22.06.2019 16:00, LikeIke2859
In which of the following is positive work done by a person on a suitcase
Answers: 1
You know the right answer?
How does the law of conservation of mass apply to this reaction: al + 3hcl → h2 + alcl3 ?
Questions in other subjects:

English, 26.02.2020 20:39


Mathematics, 26.02.2020 20:39