
Physics, 08.11.2019 03:31 goldwinner300
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe and measuring the change in temperature. for example, the combustion energy of glucose is 15.57 kj/g. when a 1.500 g sample of glucose burns in a constant volume calorimeter, the calorimeter temperature increases from 21.45 to 23.34°c. find the total heat capacity of the calorimeter (in kj/k).

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 16:00, amoryfe28p0vpwo
Lets say that an object of a certain volume has a certin mass. what happens to its mass when you double its volume?
Answers: 3

Physics, 21.06.2019 19:00, michael2737
Aman tries to push a box of mass 4 kg with a force of 5 n but the box remains stationary if the coefficient of static friction is 0.45, what is the value of the maximum force of friction?
Answers: 2


Physics, 22.06.2019 10:30, janeou17xn
Find the magnetic field a distance r from the center of a long wire that has radius a and carries a uniform current per unit area j in the positive z direction.
Answers: 2
You know the right answer?
Constant-volume calorimeters are sometimes calibrated by running a combustion reaction of known δe a...
Questions in other subjects:

Mathematics, 24.02.2020 23:58

Mathematics, 24.02.2020 23:58

Mathematics, 24.02.2020 23:58







 = 21.45
= 21.45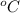
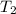 = 23.34
= 23.34


