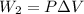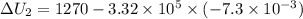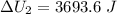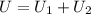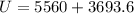Find the total change in the internal energy of a gas that is subjected to the following two-step process. in the first step the gas is made to go through isochoric heating until 5560 j of heat is transferred into the gas and its pressure is 3.32 ✕ 105 pa. in the second step it is subjected to isobaric compression until its volume decreases by 7.30 ✕ 10−3 m3 and 1270 j of heat is transferred out of the gas. what is the total change in internal energy of this gas

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 18:40, Rachaeltice8810
Ten students stand in a circle and are told to make a transverse wave. what best describes the motion of the students? each student bumps the shoulder of his or her neighbor. at the exact time, all students take a step to the right. the students skip clockwise, going in a circular motion. one at a time, each student lifts his or her hands up and then down.
Answers: 1


Physics, 22.06.2019 07:50, babbybronx
Imagine you are standing in the antarctic during the southern hemisphere summer. the sky is clear and blue. there are no clouds in the sky. your shadow is cast on the icy ground. what is the color of the shadow?
Answers: 1

Physics, 22.06.2019 15:20, alissalhenry
Arigid tank is divided into two equal parts by a partition. one part of the tank contains 3 kg of compressed liquid water at 400 kpa and 60°c while the other part is evacuated. the partition is now removed, and the water expands to fill the entire tank. determine the entropy change of water during this process, if the final pressure in the tank is 40 kpa. use steam tables.
Answers: 3
You know the right answer?
Find the total change in the internal energy of a gas that is subjected to the following two-step pr...
Questions in other subjects:






Mathematics, 24.06.2020 21:01

Biology, 24.06.2020 21:01


Mathematics, 24.06.2020 21:01

Mathematics, 24.06.2020 21:01

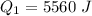
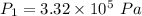
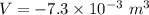 (it decreases)
(it decreases)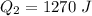
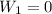
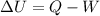
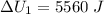 .............(1)
.............(1)