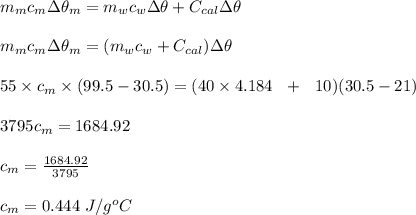Physics, 07.11.2019 01:31 jamaicalove2880
A55.0-g sample of hot metal initially at 99.5oc was added to 40.0 g of water in a styrofoam coffee cup calorimeter. the water and calorimeter were initially at 21.0oc. if the final temperature of mixture was 30.5oc, calculate the specific heat capacity of the metal. the specific heat of water is 4.184 j/(g. oc) and heat capacity of calorimeter is 10.0 j/oc.

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 11:30, alexandroperez13
With the simplified model of the eye, what corrective lens (specified by focal length as measured in air) would be needed to enable a person underwater to focus an infinitely distant object? (be careful-the focal length of a lens underwater is not the same as in air! assume that the corrective lens has a refractive index of 1.62 and that the lens is used in eyeglasses, not goggles, so there is water on both sides of the lens. assume that the eyeglasses are 1.90
Answers: 1


Physics, 22.06.2019 17:40, RayshawnBoulware
A15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°c to 175°c. what is the specific heat capacity of iron?
Answers: 1

Physics, 22.06.2019 20:00, tasniahussain21
Anika asks eva to roll a basketball and then a bowling ball to her. which requires more force to roll, and why?
Answers: 3
You know the right answer?
A55.0-g sample of hot metal initially at 99.5oc was added to 40.0 g of water in a styrofoam coffee c...
Questions in other subjects:


Mathematics, 17.02.2021 20:00

Mathematics, 17.02.2021 20:00


Mathematics, 17.02.2021 20:00

English, 17.02.2021 20:00


Mathematics, 17.02.2021 20:00

Mathematics, 17.02.2021 20:00