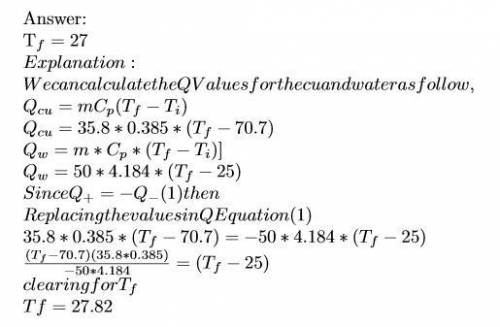
Ahot lump of 35.8 g of copper at an initial temperature of 70.7 °c is placed in 50.0 ml h2o initially at 25.0 °c and allowed to reach thermal equilibrium. what is the final temperature of the copper and water, given that the specific heat of copper is 0.385 j/(g·°c)? assume no heat is lost to surroundings.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 21:00, Kennethabrown09
Mike walks 100 meter north, then walks 30 meters south. after this, he walks another 10 meters north. what is the magnitude of his total displacement during this walk, in meters?
Answers: 2

Physics, 22.06.2019 08:30, katiemh8302
A17,250 kg rocket is pushed with a thrust of 6,450,284 n. what is the acceleration of the rocket?
Answers: 1

Physics, 22.06.2019 18:10, victoriakraus6599
A200-n force is applied to the foot-operated air pump. the return spring s exerts a 2.6-n·m moment on member oba for this position. determine the corresponding compression force c in the cylinder bd. if the diameter of the piston in the cylinder is 40 mm, estimate the air pressure generated for these conditions. state any assumptions. enter a positive number for the compression force c.
Answers: 2
You know the right answer?
Ahot lump of 35.8 g of copper at an initial temperature of 70.7 °c is placed in 50.0 ml h2o initiall...
Questions in other subjects:

Biology, 13.12.2020 09:30




Spanish, 13.12.2020 09:30

Computers and Technology, 13.12.2020 09:30

Chemistry, 13.12.2020 09:30

Mathematics, 13.12.2020 09:30