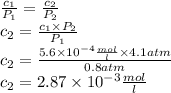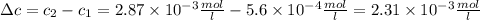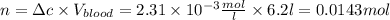The solubility of n2 in blood at 37°c and a partial pressure of 0.80 atm is 5.6 ✕ 10−4 mol·l−1. a deep-sea diver breathes compressed air with a partial pressure of n2 equal to 4.1 atm. assume that the total volume of blood in this diver's body is 6.2 l. calculate the amount of n2 gas released (in liters) when the diver returns to the surface of water, where the partial pressure of n2 is 0.80 atm. (2 sig fig)

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 09:00, jaeana
Abicycle slows down when the rider applies the brakes. what type of energy transformation is involved in this example? a. kinetic energy into heat energy b. heat energy into potential energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1

Physics, 22.06.2019 23:40, ttwright24
Which type of energy is transferred by convection currents?
Answers: 2


You know the right answer?
The solubility of n2 in blood at 37°c and a partial pressure of 0.80 atm is 5.6 ✕ 10−4 mol·l−1. a de...
Questions in other subjects:

Mathematics, 10.04.2020 18:13


Mathematics, 10.04.2020 18:13


Mathematics, 10.04.2020 18:13

Biology, 10.04.2020 18:13