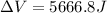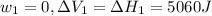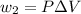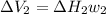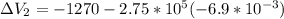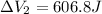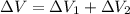Physics, 02.11.2019 07:31 chris018107
Find the total change in the internal energy of a gas that is subjected to the following two-step process. in the first step the gas is made to go through isochoric heating until 5460 j of heat is transferred into the gas and its pressure is 3.72 ✕ 105 pa. in the second step it is subjected to isobaric compression until its volume decreases by 7.50 ✕ 10−3 m3 and 1220 j of heat is transferred out of the gas. what is the total change in internal energy of this j?

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 21:40, sannai0415
Since the investigative question has two variables, you need to focus on each one separately. thinking only about the first part of the question, mass, what might be a hypothesis that would illustrate the relationship between mass and kinetic energy? use the format of "if…then…because…” when writing your hypothesis.
Answers: 1

Physics, 21.06.2019 22:00, penaabel6202
Which units can be used to measure length or distance? check all that apply. gram meter kilometer liter inch
Answers: 1


Physics, 23.06.2019 08:30, Anybody6457
The momentum of a man riding his bicycle downhill can be calculated. the bicycle and the man have a combined mass of 40 kg. the velocity of the bicycle is 10 m/s. calculate the momentum.
Answers: 1
You know the right answer?
Find the total change in the internal energy of a gas that is subjected to the following two-step pr...
Questions in other subjects:


History, 31.03.2020 21:48

English, 31.03.2020 21:48


English, 31.03.2020 21:48

Mathematics, 31.03.2020 21:48

Mathematics, 31.03.2020 21:48

Mathematics, 31.03.2020 21:48

Mathematics, 31.03.2020 21:48

Social Studies, 31.03.2020 21:48