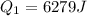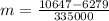Physics, 01.11.2019 01:31 makayladurham19
Two 84.5 g ice cubes are dropped into 30 g of water in a glass. if the water is initially at a temperature of 50 c and if the ice comes directly from the freezer at -300 c, what will be temperature of the drink when the ice and the water reach thermal equilibrium ? how much ice and how much water are present at thermal equilibrium ? ignore the heat capacity of the glass and heat transferred to and from the environment.

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 02:00, ghwolf4p0m7x0
In which of the following cases is work being done on an object? question 2 options: pushing against a locked door carrying a box down a corridor pulling a trailer up a hill suspending a heavy weight with a strong
Answers: 1

Physics, 22.06.2019 13:50, demitae8839
9.98 kg of r-134a at 300 kpa fills a rigid container whose volume is 14 l. determine the temperature and total enthalpy in the container. the container is now heated until the pressure is 600 kpa. determine the temperature and total enthalpy when the heating is completed. use data from the steam tables.
Answers: 1

Physics, 22.06.2019 16:00, chamyaparker
What is the freezing point of radiator fluid that is 50% antifreeze by mass? kf for water is 1.86 ∘c/m.
Answers: 3
You know the right answer?
Two 84.5 g ice cubes are dropped into 30 g of water in a glass. if the water is initially at a tempe...
Questions in other subjects:

Spanish, 13.05.2021 05:00



Mathematics, 13.05.2021 05:00