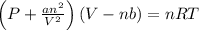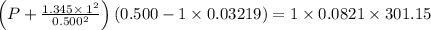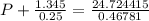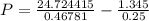Physics, 30.10.2019 00:31 miguel454545
If 1.00 mol of argon is placed in a 0.500-l container at 28.0 ∘c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 05:00, mariamalakozay603
Aperson standing in a canoe exerts a force of 700 n to throw an anchor over the side. find the acceleration of the canoe if the total mass of the canoe and the person is 100 kg?
Answers: 1

Physics, 22.06.2019 05:10, delawdermia27
Which diagram correctly demonstrates the various forces acting on a ball moving horizontally with some speed?
Answers: 2

Physics, 22.06.2019 06:00, Bengynease2598
What will a positive and a negative charge do if they are separated from each other?
Answers: 3

Physics, 22.06.2019 10:30, janeou17xn
Find the magnetic field a distance r from the center of a long wire that has radius a and carries a uniform current per unit area j in the positive z direction.
Answers: 2
You know the right answer?
If 1.00 mol of argon is placed in a 0.500-l container at 28.0 ∘c , what is the difference between th...
Questions in other subjects:



Medicine, 20.04.2020 17:14



Mathematics, 20.04.2020 17:14

Mathematics, 20.04.2020 17:14

Spanish, 20.04.2020 17:14


Mathematics, 20.04.2020 17:14