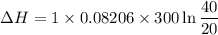One mole of a gas is placed in a closed system with a 20 l vessel initially at t = 300 k. the vessel is then isothermally expanded to 40 l. the gas follows the equation of state: p = rt/v + a/v2 where a = 240 l2 · atm/mol2 and r = 0.08206 l · atm/ mol · k. a. derive an expression relating (dh/dv)t to measurable properties. b. find dh for the gas in this process.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 22:00, treytonmesser
•• al and bert are jogging side-by-side on a trail in the woods at a speed of 0.75 m/s. suddenly al sees the end of the trail 35 m ahead and decides to speed up to reach it. he accelerates at a constant rate of 0.50 m/s2, while bert continues on at a constant speed. (a) how long does it take al to reach the end of the trail? (b) once he reaches the end of the trail, he immediately turns around and heads back along the trail with a constant speed of 0.85 m/s. how long does it take him to meet up with bert? (c) how far are they from the end of the trail when they meet?
Answers: 3

Physics, 22.06.2019 09:00, KitKat22Rose9
What is a possible result of higher air temperature caused by global warming
Answers: 1

Physics, 22.06.2019 12:30, morgan3346
Write a full page that sumerizes thermodynamics it’s from the website visionlearnig
Answers: 1

Physics, 22.06.2019 14:00, astigall4272
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1
You know the right answer?
One mole of a gas is placed in a closed system with a 20 l vessel initially at t = 300 k. the vessel...
Questions in other subjects:



Mathematics, 04.05.2021 01:00

Mathematics, 04.05.2021 01:00


Mathematics, 04.05.2021 01:00




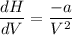
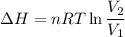 (ΔU=0)
(ΔU=0)