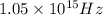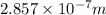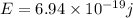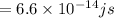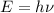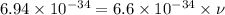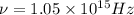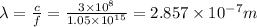Titanium metal requires a photon with a minimum energy of 6.94×10^−19j to emit electrons.
(a)...

Titanium metal requires a photon with a minimum energy of 6.94×10^−19j to emit electrons.
(a)-what is the minimum frequency of light necessary to emit electrons from titanium via the photoelectric effect? express your answer using three significant figures.
(b)- what is the wavelength of this light? express your answer using three significant figures.

Answers: 3


Other questions on the subject: Physics



Physics, 22.06.2019 11:50, taylorannsalazar
Select all that applywhat are some basic resources a family is expected to provide for children? educationclothesspending
Answers: 2

Physics, 22.06.2019 12:10, zoebtharpe
Does anyone have the answers to online physics course plato course physics, semester a v3.0
Answers: 2
You know the right answer?
Questions in other subjects:



Biology, 07.09.2019 23:20

Chemistry, 07.09.2019 23:20


Mathematics, 07.09.2019 23:20

Mathematics, 07.09.2019 23:20



Mathematics, 07.09.2019 23:20