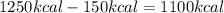1. un gas está encerrado en un cilindro ajustado con un pistón ligero sin fricción y se mantiene a presión atmosférica. cuando se agregan 1250 kcal de calor al gas, se observa que el volumen aumenta lentamente de 12.0 a 18.2 m3. calcule a) el trabajo realizado por el gas y b) el cambio en energía interna del gas.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 23:30, izzyisawesome5232
The pressure, volume, and temperature of a mole of an ideal gas are related by the equation pv = 8.31t, where p is measured in kilopascals, v in liters, and t in kelvins. use differentials to find the approximate change in the pressure if the volume increases from 14 l to 14.6 l and the temperature decreases from 375 k to 370 k. (round the answer to two decimal places.)
Answers: 3

Physics, 22.06.2019 01:30, ayalat9596
An object of dimensions 50 cm x 40 cm x 0.20 cm has a mass 60g. find its density in g/cm3 and kg/ m3
Answers: 1

Physics, 22.06.2019 02:10, lolsmaster3951
Which statement correctly describes the relationship between frequency and wavelength?
Answers: 2

Physics, 22.06.2019 15:10, Pookaapoo8832
When electrons are added to the outermost shell of a carbon atom, it forms--an anion that has a larger anion that has a smaller cation that has a larger cation that has a smaller radius.
Answers: 3
You know the right answer?
1. un gas está encerrado en un cilindro ajustado con un pistón ligero sin fricción y se mantiene a p...
Questions in other subjects:


Physics, 03.07.2019 00:00



Physics, 03.07.2019 00:00





Biology, 03.07.2019 00:00

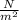
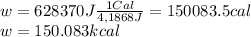 J
J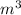
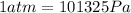
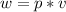 Δ
Δ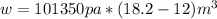
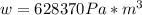
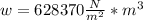
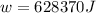
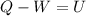 Δ
Δ