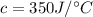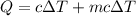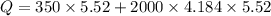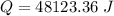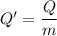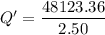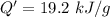Physics, 17.10.2019 20:20 awesomegrill
When 2.50 g of a certain hydrocarbon was completely combusted in a "bomb (constant-volume) calorimeter" with a heat capacity (excluding water) of 350 j/°c and which contained 2.00 liters of water (density = 1.00 g/ml and specific heat capacity = 4.184 j/°c•g), the resulting temperature change was measured to be 5.52°c. calculate the thermal energy (in kj) released per gram of hydrocarbon combusted. (1) 48.1 kj/g (2) 0.773 kj/g (3) 19.2 kj/g (4) 18.5 kj/g (5) 46.2 kj/g

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 10:10, Fionaauggies
How will the system respond to a temperature increase?
Answers: 1

Physics, 22.06.2019 11:00, kylieweeks052704
A0.580-kg rock is tied to the end of a string and is swung in a circle with a radius of 0.500 meters. the velocity of the rock is 4.50 m/s. what is the centripetal force acting on the rock? 15.5 n 5.22 n 69.8 n 23.5 n
Answers: 1


Physics, 23.06.2019 01:40, hinacat87
When you see distant streetlights through smog, they look dimmer and redder than they do normally. but when you see the same streetlights through fog or falling snow, they look dimmer but not redder. use your knowledge of the interstellar medium to discuss the relative sizes of the particles in smog, fog, and snowstorms compared to the wavelength of light.
Answers: 3
You know the right answer?
When 2.50 g of a certain hydrocarbon was completely combusted in a "bomb (constant-volume) calorimet...
Questions in other subjects:



English, 09.03.2021 01:10

Mathematics, 09.03.2021 01:10

Mathematics, 09.03.2021 01:10

English, 09.03.2021 01:10

Chemistry, 09.03.2021 01:10



History, 09.03.2021 01:10