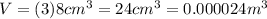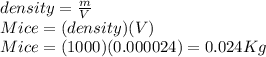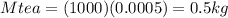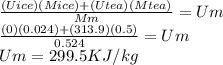Physics, 14.10.2019 17:30 guadalupemarlene2001
Suppose you are making yourself 0.50 liters of tea but its temperature is 75°c, which is too hot for you to drink. in order to cool its temperature, you immerse three ice cubes of the same dimension as the one in part b) in your tea. if the energy used to melt the ice came solely from the internal energy (i. e. temperature) of the tea, what would the reduced temperature of your tea be (where you may assume tea is made up entirely of water)? for simplicity, in this class you may generally use a value of 1000 kg/m3 for the density of water (although density, like many of the properties of water used in this problem have a relatively small temperature dependence).

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 07:30, anonymous1813
Some material consisting of a collection of microscopic objects is kept at a high temperature. a photon detector capable of detecting photon energies from infrared through ultraviolet observes photons emitted with energies of 0.3 ev, 0.5 ev, 0.8 ev, 2.0ev, 2.5ev, and 2.8ev. these are the only photon energies observed. (a) draw and label a possible energy-level diagram for one of the microscopic objects, which has four bound states. on the diagram, indicate the transitions corresponding to the emitted photons. explain briefly. (b) would a spring–mass model be a good model for these microscopic objects? why or why not? (c) the material is now cooled down to a very low temperature, and the photon detector stops detecting photon emissions. next, a beam of light with a continuous range of energies from infrared through ultraviolet shines on the material, and the photon detector observes the beam of light after it passes through the material. what photon energies in this beam of light are observed to be significantly reduced in intensity (“dark absorption lines”)? explain briefly.
Answers: 3


Physics, 22.06.2019 15:20, avree4722
Abag of potato chips contains 2.00 l of air when it is sealed at sea level at a pressure of 1.00 atm and a temperature of 20.0 deg c. what will be the volume of the air in the bag if you take it with you, still sealed, to the mountains where the temperature is 7.00 deg c and atmospheric pressure is 70.0 kpa? assume that the bag behaves like a balloon and that the air in the bag is in thermal equilibrium with the outside air.
Answers: 3
You know the right answer?
Suppose you are making yourself 0.50 liters of tea but its temperature is 75°c, which is too hot for...
Questions in other subjects:


History, 12.10.2020 23:01

Mathematics, 12.10.2020 23:01


Mathematics, 12.10.2020 23:01

Biology, 12.10.2020 23:01

History, 12.10.2020 23:01


History, 12.10.2020 23:01

Health, 12.10.2020 23:01