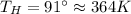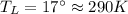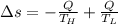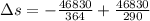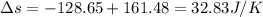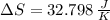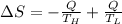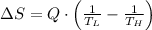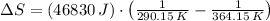Physics, 30.09.2019 23:30 noellelovebug1214
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one at a temperature of 17.0 °c. these reservoirs are brought into thermal contact long enough for 46830 j of heat to flow from the hot water to the cold water. assume that the reservoirs are large enough so that the temperatures do not change significantly. what is the total change in entropy resulting from this heat exchange between the hot water and the cold water?

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 15:30, stinematesa
A1kg cart slams into a stationary 1kg cart at 2 m/s. the carts stick together and move forward at a speed of 1 m/sl. determine whether kinetic energy was conserved in the collision. use the law of conservation of energy to explain the collision
Answers: 3

Physics, 22.06.2019 01:50, ssuereichard
Aregion of space in which a measurable gravitational force is indicated by the force exerted on a test mass is called
Answers: 1

Physics, 22.06.2019 09:40, dakshshberry
Asatellite component is in the shape of a cube with 10cm edges, has an evenly distributed mass of 2 kg and one bolt hole at each of four corners. if the maximum predicted random vibration environment is 10 grms, what bolt size would be a good choice for restraint? what other factors might drive bolt size besides force/stress?
Answers: 2

Physics, 22.06.2019 15:30, anonymous1813
What is the increase in density of a medium due to wave travel?
Answers: 2
You know the right answer?
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one a...
Questions in other subjects:




Mathematics, 10.02.2021 04:50




Mathematics, 10.02.2021 04:50

Chemistry, 10.02.2021 04:50

Mathematics, 10.02.2021 04:50