
Physics, 23.09.2019 20:00 emfastback8868
Air expands adiabatically in a piston–cylinder assembly from an initial state where p1 = 100 lbf/in.2, v1 = 3.704 ft3/lb, and t1 = 1000 °r, to a final state where p2 = 30 lbf/in.2 the process is polytropic with n = 1.4. the change in specific internal energy, in btu/lb, can be expressed in terms of temperature change as (0.171)(t2 - t1). determine the final temperature, in °r. kinetic and potential energy effects can be neglected.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 05:30, shychk04ot109f
Choose the most likely outcome of this scenario: jen decided to go bike riding without a helmet. while no one is around during her ride, she is thrown from her bike when her wheel goes into a pothole. she is not injured, but she is terrified to get back on her bike. what happens next? a. her physical health is affected even though she wasn't hurt. b. her mental and emotional health are affected because she is afraid to get back on her bike. c. her social health is affected because she is worried her friends saw the fall. d. her overall health is not affected at all by her fall.
Answers: 1

Physics, 22.06.2019 07:30, alejandro1102
The charge on a charged sphere is: a)concentrated at its centerb)distributed uniformly throughout its volumec)clustered on its centerd)distributed uniformly over its surface
Answers: 1

Physics, 22.06.2019 09:00, rhettperkins
One form of energy can be another type of energy. a. created to form b. transformed into c. destroyed and then created to form
Answers: 1

Physics, 23.06.2019 03:30, Carlyalexis77301
First to answer will be the brainliest i need the answer asap
Answers: 2
You know the right answer?
Air expands adiabatically in a piston–cylinder assembly from an initial state where p1 = 100 lbf/in....
Questions in other subjects:



Mathematics, 08.01.2021 23:20


Mathematics, 08.01.2021 23:20

Mathematics, 08.01.2021 23:20



Chemistry, 08.01.2021 23:20

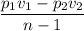
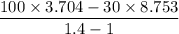
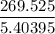 Btu/lb
Btu/lb


