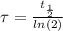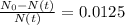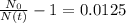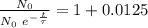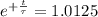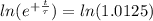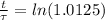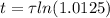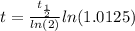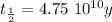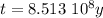The ages of rocks that contain fossils can be determined using the isotope 87rb. this isotope of rubidium undergoes beta decay with a half‑life of 4.75×1010y . ancient samples contain a ratio of 87sr to rb87 of 0.0125. given that 87sr is a stable product of the beta decay of 87rb, and assuming there was originally no 87sr present in the rocks, calculate the age of the rock sample. assume that the decay rate is constant over the relatively short lifetime of the rock compared to the half-life of 87rb.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 04:30, phyllides4930
The current in a hair dryer measures 11 amps. the resistance of the hair dryer is 12 ohms. what is the voltage?unit:
Answers: 1


Physics, 22.06.2019 08:50, dijonmckenzie3
The electronic structure or chlorine is 2.8.7 what is the electronic structure of fluorine?
Answers: 2

Physics, 22.06.2019 12:50, stephanie37766
Which changes would result in a decrease in the gravitational force btween two objects? check all that apply
Answers: 1
You know the right answer?
The ages of rocks that contain fossils can be determined using the isotope 87rb. this isotope of rub...
Questions in other subjects:


Mathematics, 27.06.2019 09:30

Mathematics, 27.06.2019 09:30

Geography, 27.06.2019 09:30






Mathematics, 27.06.2019 09:30

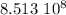 years
years 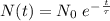
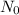 is the initial quantity of the material, and
is the initial quantity of the material, and  is the mean lifetime of the material.
is the mean lifetime of the material.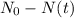
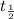 ) by the relationship
) by the relationship