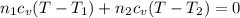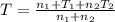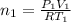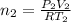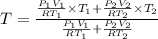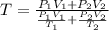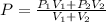Physics, 14.09.2019 07:20 jamalnellum56
Arigid adiabatic container is divided into two parts containing n1 and n2 mole of ideal gases respectively, by a movable and thermally conducting wall. their pressure and volume are p1, v1 for part 1 and p2, v2 for part 2 respectively. find the final pressure p and temperature t after the two gas reaches equilibrium. assume the constant volume specific heats of the two gas are the same.

Answers: 2


Other questions on the subject: Physics


Physics, 21.06.2019 18:30, Tierriny576
A6,600 kg train car moving at +2.0 m/s bumps into and locks together with one of mass 5,400kg moving at -3.0m/s. what is their final velocity? +0.50 m/s -1.0 m/s +2.5 m/s -0.25 m/s
Answers: 3

Physics, 22.06.2019 09:00, jaeana
Abicycle slows down when the rider applies the brakes. what type of energy transformation is involved in this example? a. kinetic energy into heat energy b. heat energy into potential energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1

Physics, 22.06.2019 14:20, kaylaelaine18
What are the starting materials for nuclear fission? two small nuclei two large nuclei a neutron and a large nucleus a neutron and a small nucleus
Answers: 2
You know the right answer?
Arigid adiabatic container is divided into two parts containing n1 and n2 mole of ideal gases respec...
Questions in other subjects:


Mathematics, 03.12.2020 01:20

Geography, 03.12.2020 01:20

Mathematics, 03.12.2020 01:20




History, 03.12.2020 01:20


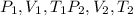
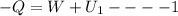

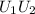 change in internal Energy of gas
change in internal Energy of gas