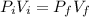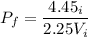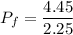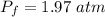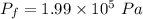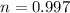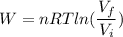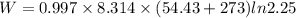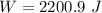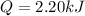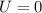Amass of 222g of helium gas at an initial temperature of 54.43°c and at an initial absolute pressure of 4.45 atm undergoes an isothermal expansion until its volume increases by a factor of 2.25. (a) what is the final pressure? (pa)
(b) how much work is done on the gas?
(c) how much heat does the gas absorb?
(d) what is the change in the total internal energy of the gas?

Answers: 2


Other questions on the subject: Physics


Physics, 22.06.2019 02:30, 22emilyl530
Agas contained within a piston-cylinder assembly undergoes three processes in series: process 12: compression with pv= constant from 1 bar and 1 liter to 4 bar. process 23: constant pressure expansion to 1 liter. process 31: constant volume calculate the pressure and volume at each state, and sketch the processes on a p-vdiagram labeled with pressure and volume values at each numbered stat
Answers: 2

You know the right answer?
Amass of 222g of helium gas at an initial temperature of 54.43°c and at an initial absolute pressure...
Questions in other subjects:


Mathematics, 12.03.2020 20:44





Mathematics, 12.03.2020 20:44

History, 12.03.2020 20:44