Physics, 05.09.2019 17:10 zahradawkins2007
You have 1.50 kg of water at 28.0∘ in an insulated container of negligible mass. you add 0.700 kg of ice that is initially at -21.0 ∘c. assume that no heat exchanges with the surroundings.
(a) after the thermal equilibrium has been reached, has all the ice melted?
(b) if all of the ice is melted, what is the final temperature of the water in the container? if some ice remains, what is the final temperature of the water in the container, and how much ice remains?

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 19:30, noberoger2780
Asun-sized star will spend most of its lifetime as a: white dwarf red giant protostar main-sequence star
Answers: 1


Physics, 22.06.2019 21:30, Hockeypro1127
The three classes of rocks are sedimentary, metamorphic, and igneous. how are rocks classified into one of these three groups?
Answers: 1

Physics, 23.06.2019 00:20, nadiareese
When can a theory be modified if a new type of technology allows for new observations that raise new questions? a-immediately, while the questions about the theory are being asked b-after new hypotheses related to the theory are tested in experiments c-only after the new observations disprove all parts of the theory d-while scientists begin to think about how the theory could improve
Answers: 1
You know the right answer?
You have 1.50 kg of water at 28.0∘ in an insulated container of negligible mass. you add 0.700 kg of...
Questions in other subjects:



Biology, 12.11.2019 18:31

Social Studies, 12.11.2019 18:31


Chemistry, 12.11.2019 18:31



History, 12.11.2019 18:31

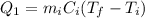
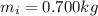 is the mass of the ice
is the mass of the ice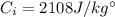 is the heat specific capacity of ice
is the heat specific capacity of ice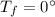 is the final temperature of the ice
is the final temperature of the ice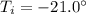 is the initial temperature
is the initial temperature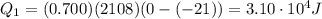
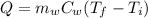
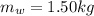 is the mass of the water
is the mass of the water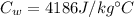 is the heat specific capacity of water
is the heat specific capacity of water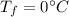 is the final temperature at equilibrium
is the final temperature at equilibrium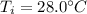 is the initial temperature of the water
is the initial temperature of the water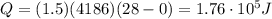
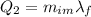
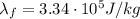 is the latent heat of fusion of ice
is the latent heat of fusion of ice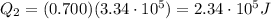
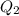 is larger than Q: this means that not all the ice melts.
is larger than Q: this means that not all the ice melts. 
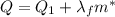
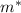 is the mass of ice that has melted. Solving for this variable, we find:
is the mass of ice that has melted. Solving for this variable, we find: