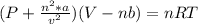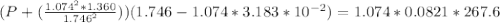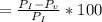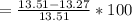Physics, 05.09.2019 16:10 skgoldsmith
According to the ideal gas law, a 1.074 mol sample of oxygen gas in a 1.746 l container at 267.6 k should exert a pressure of 13.51 atm. what is the percent difference between the pressure calculated using the van der waals' equation and the ideal pressure? for o2 gas, a = 1.360 l2atm/mol2 and b = 3.183×10-2 l/mol.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 12:30, ayoismeisjjjjuan
Cathy is pedaling down a steep hill on her bicycle and wants to be able to coast up the next hill, which is 25 meters high, without pedaling. cathy and her bicycle have a mass of 75 kg. assuming her bicycle is 100% efficient, what would her speed have to be?
Answers: 3


You know the right answer?
According to the ideal gas law, a 1.074 mol sample of oxygen gas in a 1.746 l container at 267.6 k s...
Questions in other subjects:


Business, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01



Mathematics, 01.09.2020 01:01


Mathematics, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01