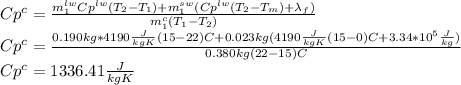A380 g metal container, insulated on the outside, holds 190 g of water in thermal equilibrium at 22°c. a 23 g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15°c. assume there is no heat exchange with the surroundings. for water, the specific heat capacity is 4190 j/kg ∙ k and the heat of fusion is 3.34 × 105 j/kg. the specific heat capacity for the metal is closest to:
3440 j/kg ∙ k
2340 j/kg ∙ k
1340 j/kg ∙ k
840 j/kg ∙ k
2840 j/kg ∙ k

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 08:50, dijonmckenzie3
The electronic structure or chlorine is 2.8.7 what is the electronic structure of fluorine?
Answers: 2

Physics, 22.06.2019 16:00, alecnewman2002
The energy produced as a result of this flow of electrons from atom to atom is called
Answers: 3

Physics, 22.06.2019 22:30, josephaciaful
Determine the net charge of the predominant form of arg at (a) ph 1.0, (b) ph 5.0, (c) ph 10.5, and (d) ph 13.5
Answers: 2
You know the right answer?
A380 g metal container, insulated on the outside, holds 190 g of water in thermal equilibrium at 22°...
Questions in other subjects:

Mathematics, 22.04.2020 02:17



Social Studies, 22.04.2020 02:17






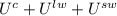




 .
.
 and
and 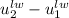 can be written as
can be written as 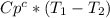 and
and 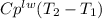 respectively. So, our energy balance comes up:
respectively. So, our energy balance comes up:
 is an energy change which involves a phase change, so it can't be calculated as the others. However, this internal specific energy change can be calculated by steps, first melting the ice and then heating the water. When melting the ice the associated energy is the heat of fusion:
is an energy change which involves a phase change, so it can't be calculated as the others. However, this internal specific energy change can be calculated by steps, first melting the ice and then heating the water. When melting the ice the associated energy is the heat of fusion: 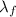 and when heating the liquid water the associated energy is (as it does not involve a phase change)
and when heating the liquid water the associated energy is (as it does not involve a phase change)  where
where 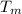 is the melting temperature. Bear in mind that for the calculation of
is the melting temperature. Bear in mind that for the calculation of 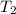 ). Then, the internal specific energy change
). Then, the internal specific energy change 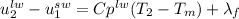
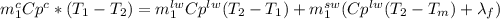
 , so let's find it:
, so let's find it: