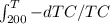Physics, 13.08.2019 02:30 jones03riley
You have two identical solid objects (same mass mand specific heat c).they differ only in that one is at a temperature th0= 450 k and the other at tc0=200 k. there is no phase transition between these two temperatures. you run a heat engine between themuntil they are both at the same final temperature. to do the maximum amount of work, what would be that final temperature?

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 07:00, lujaynsparkles
Which articulations allow for greatest range of motion?
Answers: 1

Physics, 22.06.2019 13:20, Jbutler15
This energy transformation diagram represents the energy of a skateboarder moving along a half-pipe. as she skates toward the top of the half-pipe, her original kinetic energy is converted to potential energy and friction. how much of the energy is potential?
Answers: 3

Physics, 22.06.2019 17:30, herbal420medici
Ethanol has a heat of vaporization of 38.56 kj/mol and a normal boiling point of 78.4 c. what is the vapor pressure of ethanol at 14 c?
Answers: 3

Physics, 22.06.2019 18:50, naiomireyes74p2aybs
What accounts for an increase in the temperature of a gas that is kept at constant volume? a. energy has been removed as heat from the gas. b. energy has been added as heat to the gas. c. energy has been removed as work done by the gas. d. energy has been added as work done by the gas.
Answers: 2
You know the right answer?
You have two identical solid objects (same mass mand specific heat c).they differ only in that one i...
Questions in other subjects:

Mathematics, 21.12.2021 15:40

Social Studies, 21.12.2021 15:40


Mathematics, 21.12.2021 15:40

Social Studies, 21.12.2021 15:50




Mathematics, 21.12.2021 15:50

Mathematics, 21.12.2021 15:50

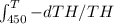 =
=