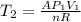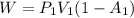Physics, 02.08.2019 21:30 girlgirl7230
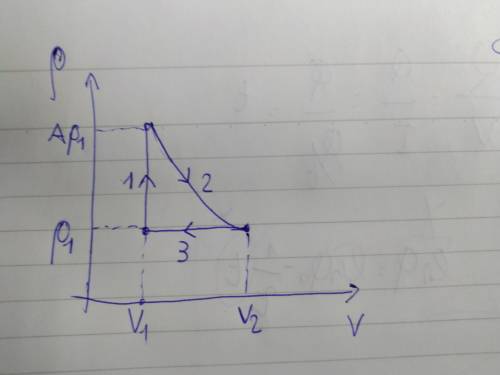
Amonatomic ideal gas has pressure p1 and temperature t1. it is contained in a cylinder of volume v1 with a movable piston, so that it can do work on the outside world. consider the following three-step transformation of the gas: the gas is heated at constant volume until the pressure reaches ap1 (where a> 1). the gas is then expanded at constant temperature until the pressure returns to p1. the gas is then cooled at constant pressure until the volume has returned to v1.
it may be to sketch this process on the pvplane.
how much heat q1 is added to the gas during step 1 of the process?
express the heat added in terms of p1, v1, and a.
how much work w2 is done by the gas during step 2?
express the work done in terms of p1, v1, and a.
how much work w3 is done by the gas during step 3?
if you've drawn a graph of the process, you won't need to calculate an integral to answer this question.
express the work done in terms of p1, v1, and a.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 12:00, louieknown
Suppose a wire with a current is pushed upward by a magnetic field. how would the wire move if the direction of the current reversed? explain your answer.
Answers: 1


Physics, 22.06.2019 18:00, Zaydblackwood06
The photo shows sugar dissolved into a solution with excess sugar at the bottom of the jar this type of solution is classified as a. unsaturated b. compound c. saturated d. plasma
Answers: 1

Physics, 23.06.2019 02:10, naomicervero
What is the quantity which is measured by the area occupied below the velocity- time graph?
Answers: 2
You know the right answer?
Amonatomic ideal gas has pressure p1 and temperature t1. it is contained in a cylinder of volume v1...
Questions in other subjects:





Mathematics, 06.10.2019 15:00

Mathematics, 06.10.2019 15:00




Mathematics, 06.10.2019 15:00

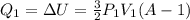
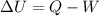
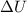 is the change in internal energy
is the change in internal energy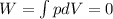
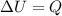
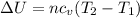 (1)
(1)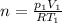 (2)
(2)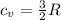 is the specific heat at constant volume
is the specific heat at constant volume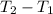 is the change in temperature. The temperature T2 can calculate again by using the ideal gas law at the new conditions of the gas, after its pressure has reached Ap1:
is the change in temperature. The temperature T2 can calculate again by using the ideal gas law at the new conditions of the gas, after its pressure has reached Ap1: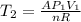 (3)
(3)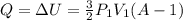
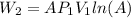
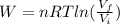 (4)
(4)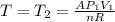 is the constant temperature of the process, found in the previous part
is the constant temperature of the process, found in the previous part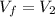 is the final volume, which can be found again by using the ideal gas law:
is the final volume, which can be found again by using the ideal gas law: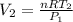
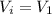 is the initial volume
is the initial volume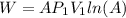
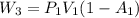
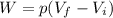
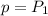 is the constant pressure of the process
is the constant pressure of the process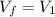 is the final volume
is the final volume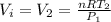 is the initial volume
is the initial volume