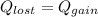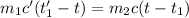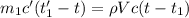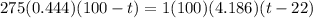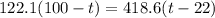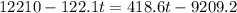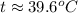Physics, 02.08.2019 19:10 littlemoneyh
A275-g sample of nickel at 100.0°c is placed in 100.0 ml of water at 22.0°c. what is the final temperature of the water? assume that no heat is lost to or gained from the surroundings. specific heat capacity of nickel = 0.444 j/(g·k)

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 06:30, marciamwilliamp31fz6
The energy of a photon was found to be 3.38 × 10–19 j. planck’s constant is 6.63 × 10–34 j • s. which color of light corresponds to this photon?
Answers: 2

Physics, 22.06.2019 12:30, cardsqueen
Consider a hydrogen atom in the ground state. what is the energy of its electron? =e= jj now consider an excited‑state hydrogen atom. what is the energy of the electron in the =5n=5 level? =e5= j
Answers: 3

Physics, 22.06.2019 23:00, destinybonmer
1which body contains the majority of the mass in the solar system earth jupiter the moon the sun 2 what keeps the objects in the solar system such as planets orbiting around the sun the force of gravity the galaxy exerts on all objects within it including the solar system the great distance between the sun and the planets the sun's nuclear fusion which acts on each object the gravitational force between the sun and each object in the solar system
Answers: 2

Physics, 23.06.2019 00:00, jazminemendezhidalgo
If water vapor with the mass of 35.0 grams is cooled and condensed into water in a closed system such as a closed glass jar, what is the mass of the water after the water vapor completely condenses? a) 34.5 grams b) 35.0 grams c) 35.5 grams d) the mass cannot be determined without knowing the volume
Answers: 2
You know the right answer?
A275-g sample of nickel at 100.0°c is placed in 100.0 ml of water at 22.0°c. what is the final tempe...
Questions in other subjects:



Health, 12.09.2019 22:30



Mathematics, 12.09.2019 22:30