Physics, 02.08.2019 18:10 babyface1686
At 25°, the free energy of formation of gaseous water is -229 kj/mol. calculate δg for the following reaction if the hydrogen is supplied at 4.00 atm and the oxygen is supplied at 3.00 atm, while the water produced is at 1.00 atm pressure.

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 11:50, kaylallangari2145
Amoving electron has kinetic energy k1. after a net amount of work w has been done on it, the electron is moving one-quarter as fast in the opposite direction. (a) find w in terms of k1. (b) does your answer depend on the final direction of the electron's motion?
Answers: 2


Physics, 22.06.2019 23:30, ghari112345
How is the ideal mechanical advantage of a wheel and axle calculated?
Answers: 1
You know the right answer?
At 25°, the free energy of formation of gaseous water is -229 kj/mol. calculate δg for the following...
Questions in other subjects:


English, 11.06.2021 23:50

Mathematics, 11.06.2021 23:50



Mathematics, 11.06.2021 23:50

Mathematics, 11.06.2021 23:50

Spanish, 11.06.2021 23:50


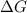 for the reaction is -467.595 kJ/mol
for the reaction is -467.595 kJ/mol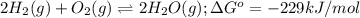
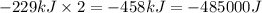 (Conversion factor: 1kJ = 1000J)
(Conversion factor: 1kJ = 1000J)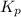 for the given reaction:
for the given reaction: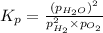
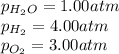
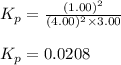
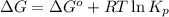
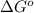 = Standard Gibbs' free energy change of the reaction = -458000 J
= Standard Gibbs' free energy change of the reaction = -458000 J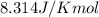
![25^oC=[25+273]K=298K](/tpl/images/0162/6752/df1f6.png)